Abstract
Background: Methodological advances and improvements in supportive care have increased the number of patients eligible for allogenic hematopoietic cell transplantation (HCT), yet the procedure is a highly technical process that remains available only at select centers in the United States. For this reason, many patients live at great distances from their HCT center, and the need for close and specialized follow-up in the months after the procedure can cause a substantial burden on familial finances, caregivers, and quality of life (QOL). One way of potentially ameliorating these effects is to allow some post-HCT care to be provided by non-HCT local hematologic oncologists closer to where patients live. Such a "shared care" model could reduce patient-centered burdens post-HCT; however, it is not known if, given its complexity, post-HCT care can be safely shared with local providers without compromising HCT outcomes.
Methods: From December of 2017 to December of 2021, we conducted a randomized controlled trial to assess the effectiveness of a post-HCT shared care program. We enrolled patients referred to Dana-Farber Cancer Institute (DFCI) for HCT from eight local sites: Lifespan (RI); Dartmouth-Hitchcock (NH); New York Oncology Hematology (NY); Northern Light Medical Center (ME), New England Cancer Specialists (ME), and three DFCI Community Satellites (in MA and NH). Patients were approached about randomization to Shared Care vs. Usual Care when they first presented to DFCI for HCT consultation, and randomized 1:1 after HCT consent, stratified by referring site. The Shared Care model involved four care delivery strategies to allow patients to be safely seen locally after HCT: a formal online care coordination plan, patient engagement and education, local physician engagement and education (including yearly face-to-face training with DFCI transplant physicians), and a shared patient-physician-transplanter web portal and communication platform. The study design included alternating visits between the local site and DFCI through day 100 in the Shared Care arm, and visits exclusively at DFCI in the Usual Care arm. The two co-primary outcomes were non-relapse mortality at day 100 and QOL at day 180 (scores on the FACT-BMT and EORTC QLQ-C30). We also assessed overall survival (OS) and QOL at day 100.
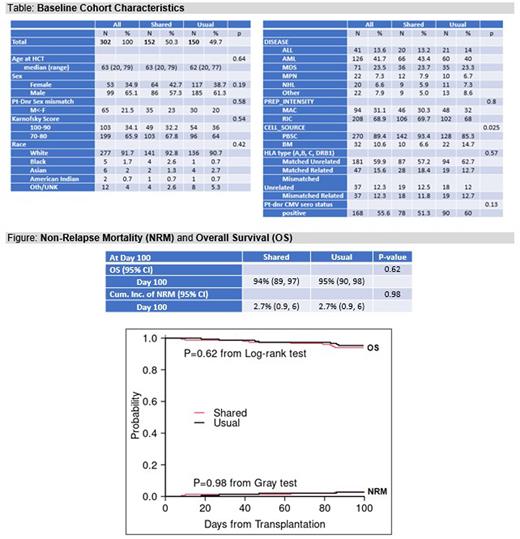
Results: At the end of the study period, 326 patients who consented for allogeneic HCT had enrolled and 2 withdrew. Of the remaining 324, 302 underwent HCT, with 152 randomized to Shared Care and 150 to Usual Care. The median age at HCT was 63, with 65.1% males and 91.7% White. Baseline characteristics were similar except cell source, with more bone marrow in Usual Care (p=0.03; Table). The day 100 non-relapse mortality rate was 2.6% for Shared Care and 2.7% for Usual Care (p=0.98). There were 7 deaths for any cause in the Shared Care group and 9 in the Usual Care group, and the OS rate at day 100 was 94% vs 95%, respectively (p=0.62; Figure). Grade II-IV acute GVHD (p=0.60) and grade ≥3 infection (p=0.63) were both similar at day 100. The day 100 QOL survey response rate among those still alive was 60.5%; the day 180 response rate was 67.3%. There were no significant differences in QOL at day 180 on the FACT-BMT total score (p= 0.36) or QLQ-C30 global score (p=0.54), nor the various subscales of each (all p > 0.05). In contrast, at day 100, the FACT-BMT total score was significantly better in the Shared Care group (p= 0.02) compared to the Usual Care group, with the physical well-being (p=0.004), emotional well-being (p=0.04) and bone marrow transplantation subscales (p=0.007) all better as well. This pattern was also seen on the QLQ-C30 at day 100, where the global (p=0.02), emotional (0.03), cognitive (0.04), fatigue (0.01), nausea (0.01) and dyspnea (0.02) scores were all better for Shared Care.
Conclusion: As compared to Usual Care, Shared Care with local providers after HCT did not compromise day 100 non-relapse mortality, and led to improved QOL as measured by the FACT-BMT and QLQ-C30 at day 100. These data suggest that Shared Care has the potential to become a standard model for follow-up care after allogeneic HCT. Implementation will require rigor adherence to the four care delivery strategies detailed above.
Disclosures
Abel:Novartis: Consultancy. Zackon:Ontada: Current Employment; Incyte: Consultancy, Other: Research Consulting Services to Incyte; Epizyme: Consultancy; Morphosys: Consultancy. Cutler:Omeros: Consultancy; Equilium: Consultancy; Pfizer: Consultancy; Editas: Consultancy; CareDx: Consultancy; Mallinckrodt: Consultancy; CTI BioPharma: Consultancy; BMS: Consultancy; Janssen: Consultancy; Sanofi: Consultancy; Incyte: Consultancy; Deciphera: Consultancy; Jazz: Consultancy; Cimeio: Current equity holder in private company. Ho:Jazz: Research Funding; Omeros: Consultancy; Allovir: Consultancy; Alexion: Consultancy. Soiffer:Kiadis: Membership on an entity's Board of Directors or advisory committees; Juno: Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy; Be The Match/National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy; Rheos Therapeutics: Consultancy; Jazz: Consultancy; VOR Biopharma: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal